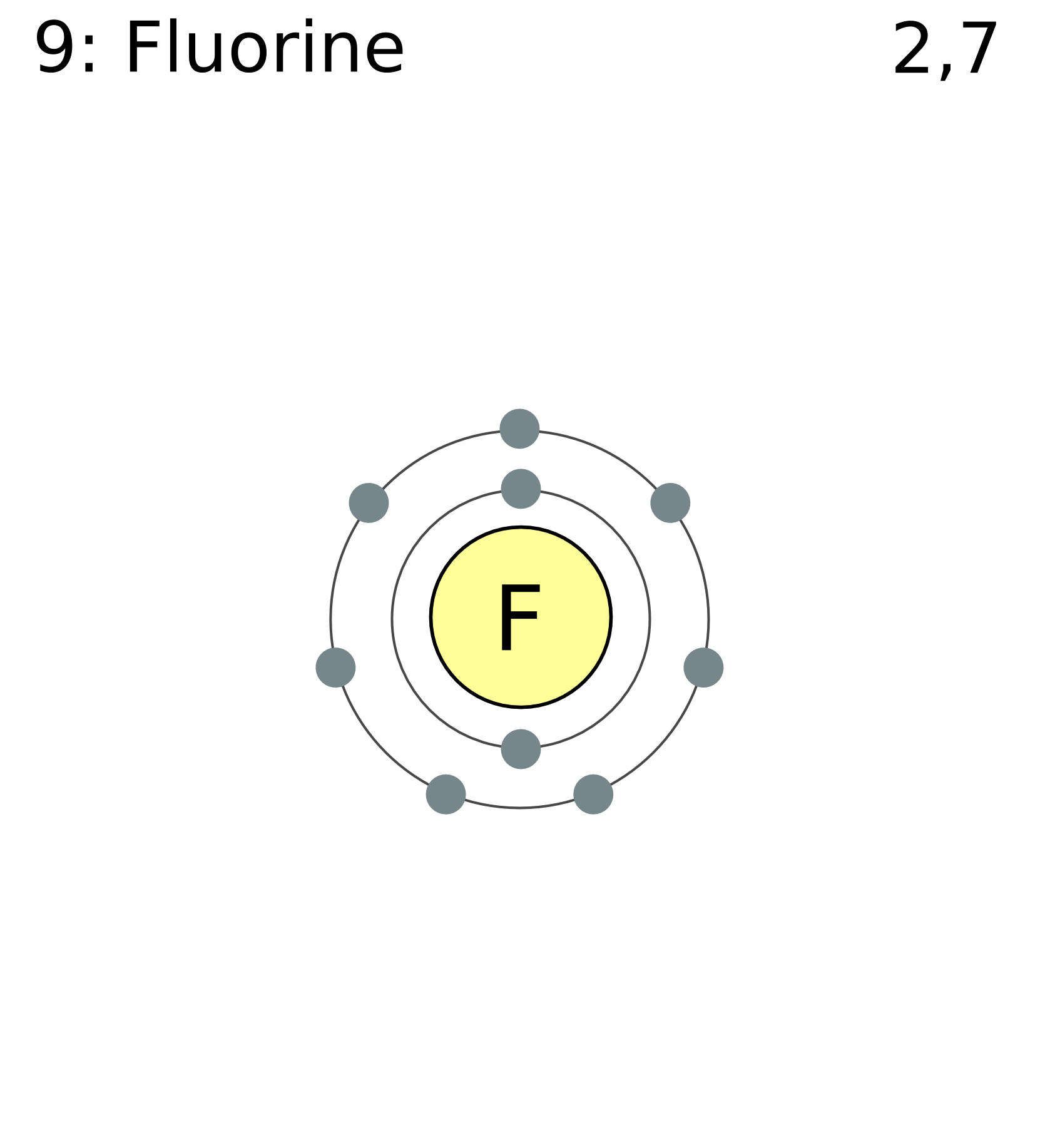
Fluorine Atom Diagram
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Fluorine atom with some simple steps. Steps to draw the Bohr Model of Fluorine atom 1. Find the number of protons, electrons, and neutrons in the Fluorine atom

Fluorine Molecule Diagram
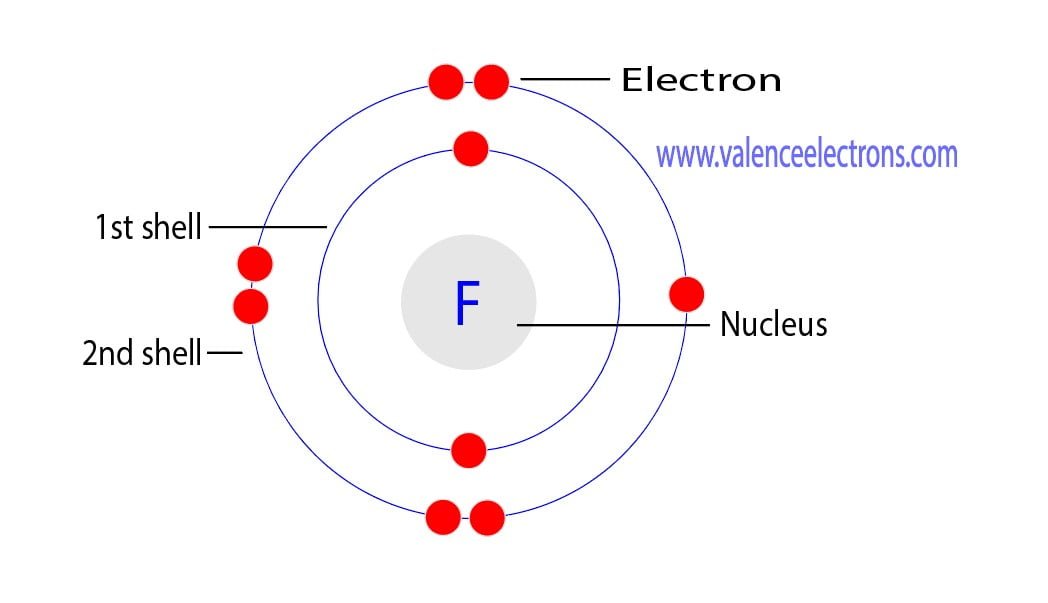
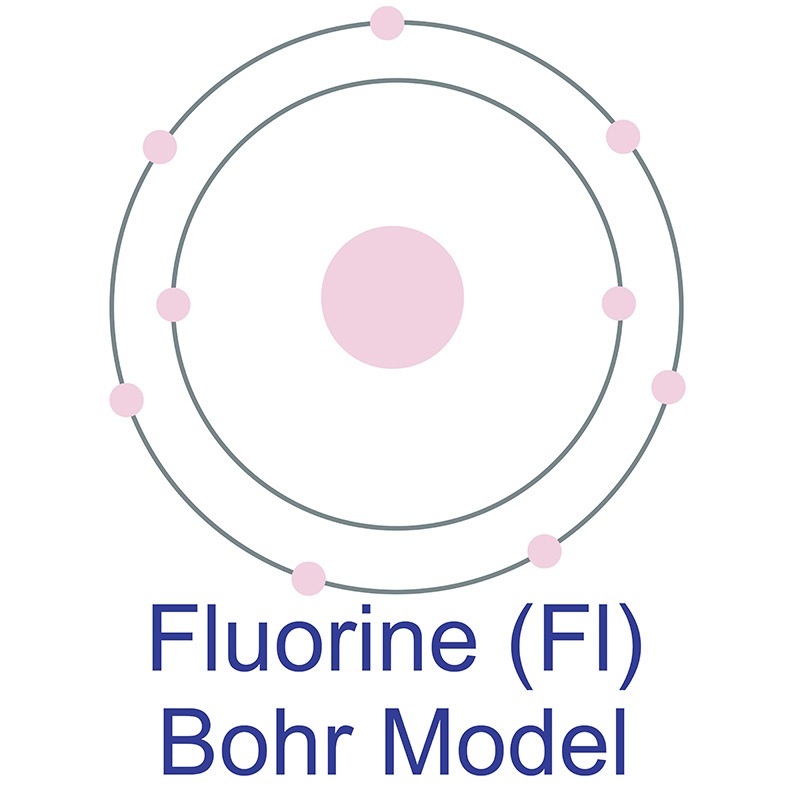
Fluorine has 2 electrons in its first shell and 7 in its second.Check me out: http://www.chemistnate.com

Fluorine atom diagram concept Stock Vector Image & Art Alamy
Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons
ShowMe fluorine
How to draw a Bohr-Rutherford Diagram? Draw a nucleus -write the number of protons and neutrons inside the nucleus. Draw orbitals around the nucleus. Represent electrons as pairs of dots in the orbitals. Draw electrons as dots on the rings that represent the energy levels. Each ring has a maximum number of electrons that it can hold.

Grade 9 BohrRutherford Diagram Fluorine YouTube
Set up the diagram. To set up the diagram, you will need a circle in the middle. This will represent the nucleus. Here you will write the number of protons and neutrons as shown below in this example using sodium (Na) Add in orbitals and electrons. In the last step you will need to draw circles around the nucleus.
bohr rutherford diagrams lithium
Schematic diagram of the Rutherford-Bohr atomic model with one orbital electron of mass m e and a finite nucleus of mass M. Both the orbital electron and the nucleus revolve about their common center-of-mass. Full size image. The following relationships will be useful in our calculations.
Fluorine Bohr model Science ShowMe
Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules
Bohr Diagram Of Flourine
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.
Fluorine Atom Bohr Model Proton Neutron Electron Illustration Stock
Knowing this information allows us to set up our Bohr-Rutherford Diagram. Let's create one using Lithium. First determine the number of protons, neutrons and electrons. Lithium. Atomic Number = 3 #p = 3 (since atomic number is 3) Atomic Mass = 7 #n = 4 (since 7 - 3 = 4) #e = 3 (since same as #p) The Bohr-Rutherford Diagram for Lithium would.
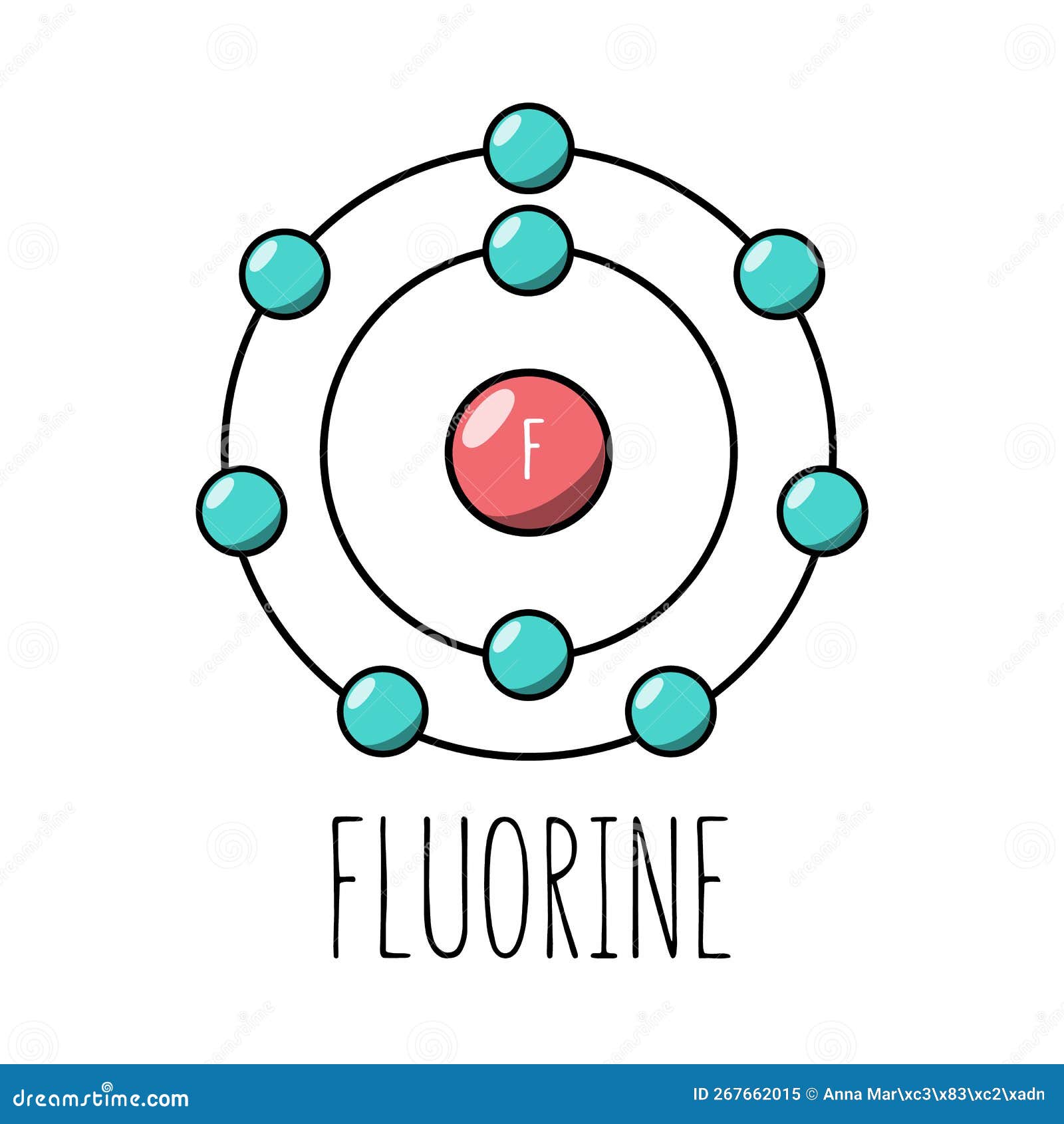
Fluorine atom Bohr model stock vector. Illustration of flat 267662015
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.

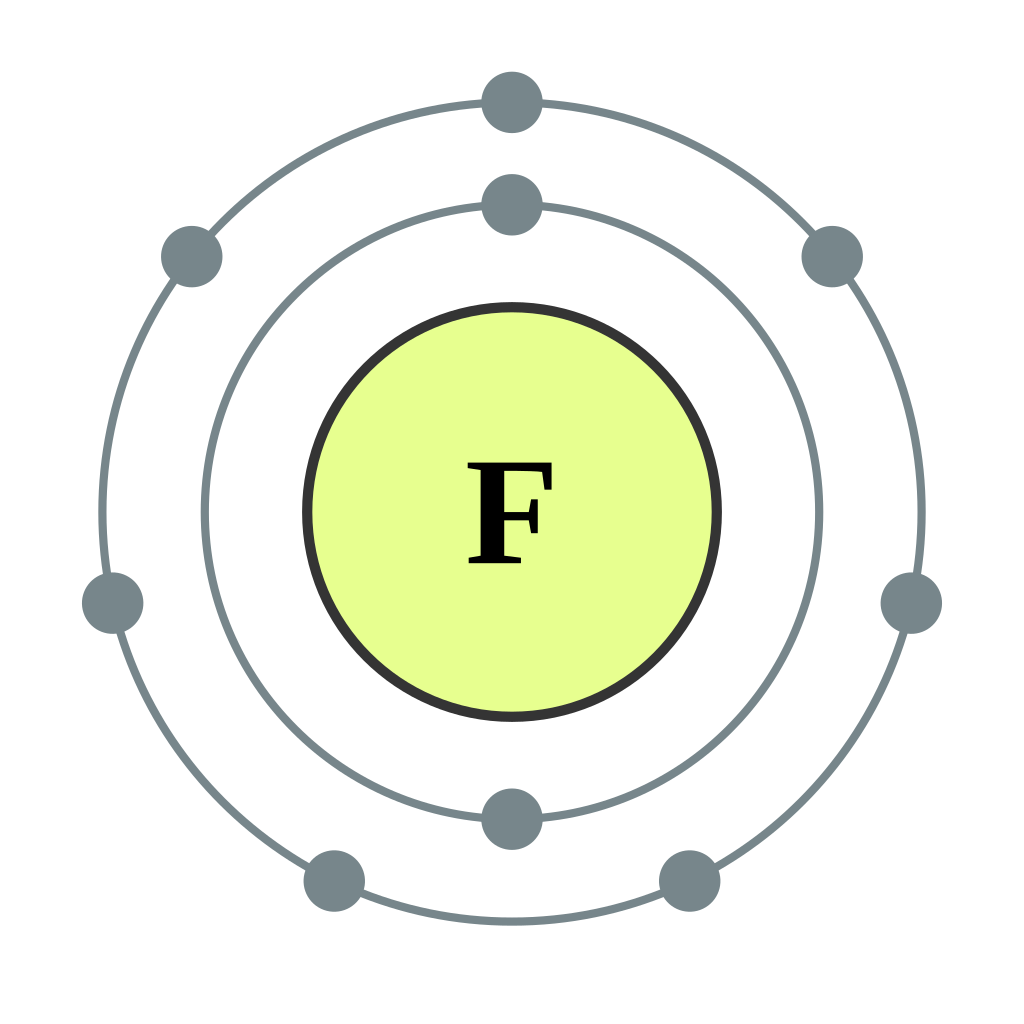
FichierElectron shell 009 Fluorine.svg Vikidia, l’encyclopédie des 8
This video expectation is to understand how the Bohr-Rutherford was designed and effectively use a template to draw the Bohr-Rutherford diagram of the first.

Fluorine Electron Configuration With Full Orbital Diagram
In 1913 Niels Bohr combined Rutherford's concept of the nuclear atom with Planck's idea of the quantized nature of the radiative process and developed, from first principles, an atomic model that successfully deals with one-electron structures like the hydrogen atom and one-electron ions such as singly ionized helium, doubly ionized lithium, etc. forming a hydrogen-like or hydrogenic.
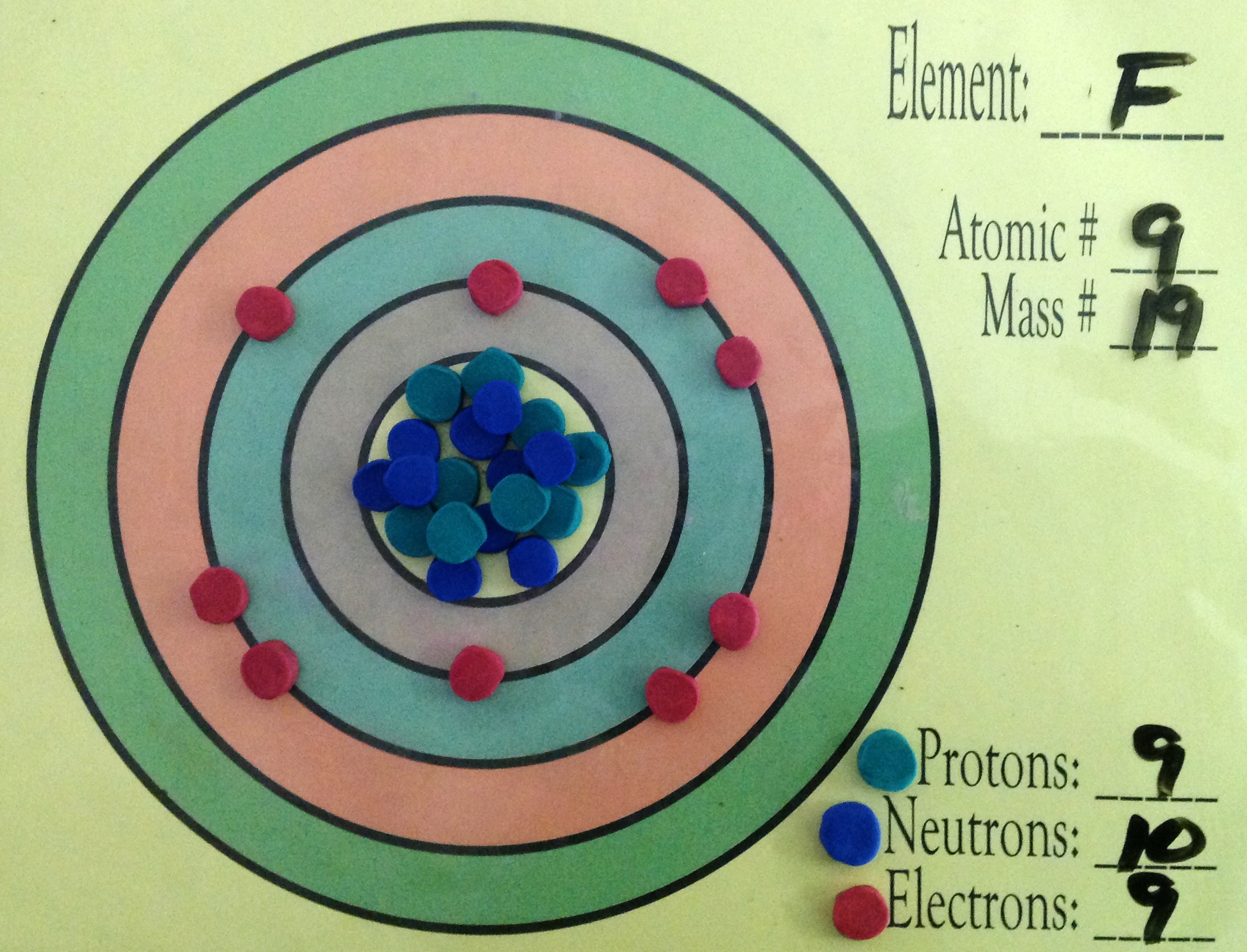
Fluorine Atom Diagram
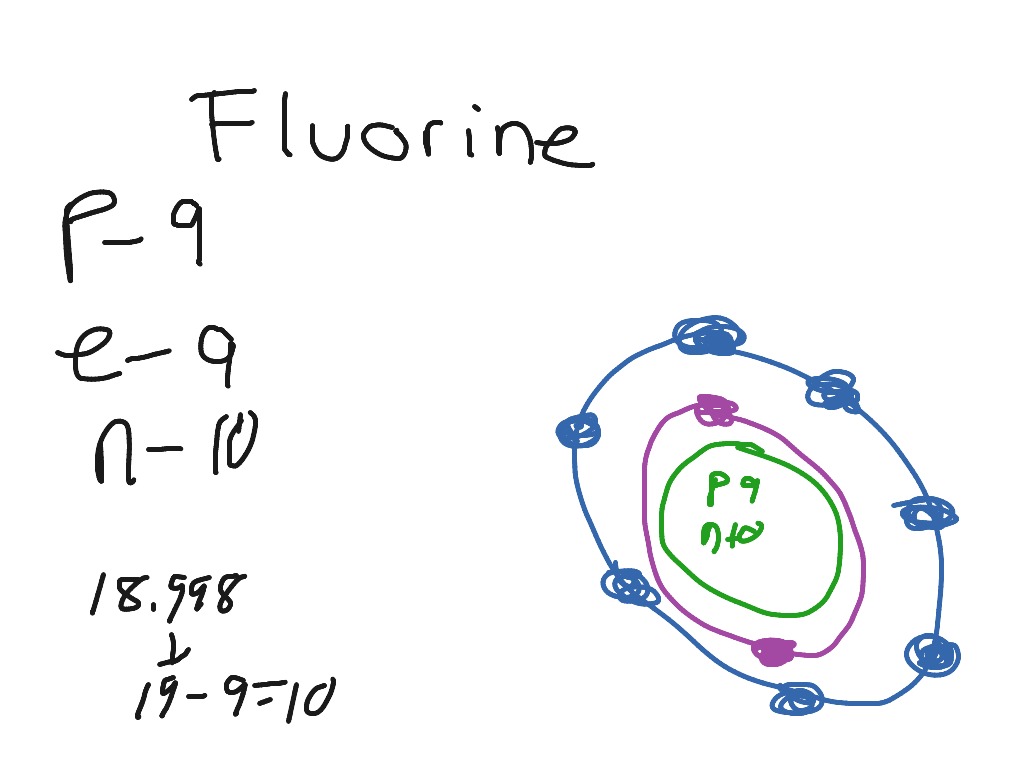
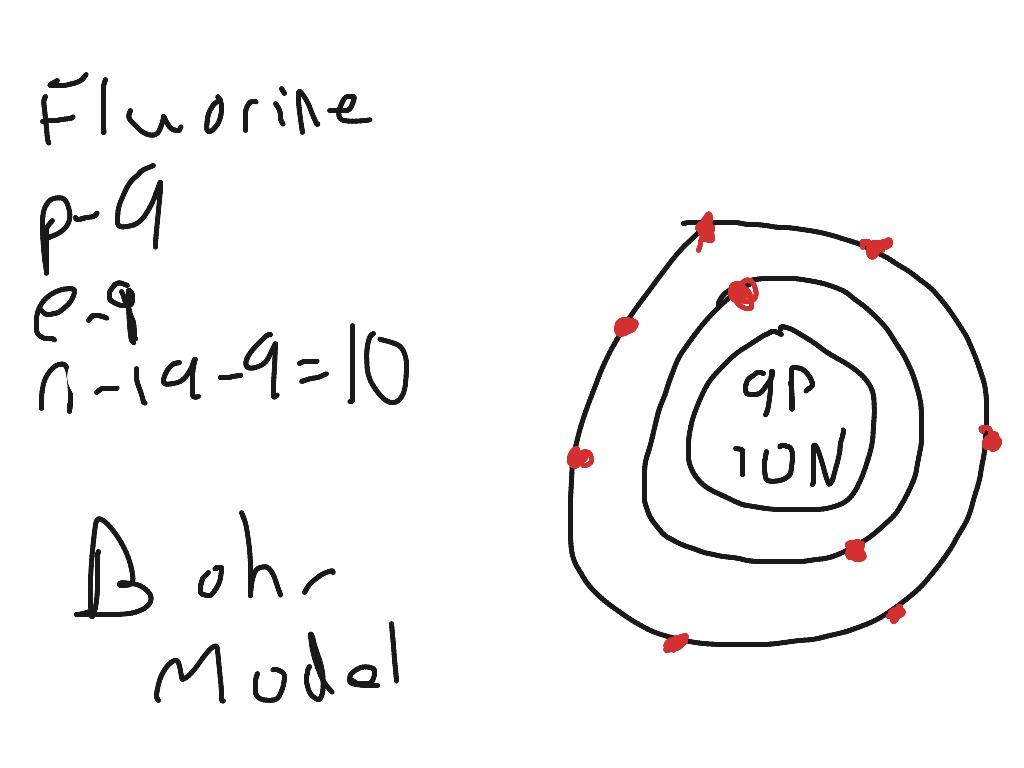
This is the Bohr-Rutherford Diagram for Fluorine (Atomic Number 9). Fluorine is the ninth element of the Periodic Table. As shown, Fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). Thus, its Atomic Mass is 19. Fluorine also has nine total electrons -- two electrons in Orbit 1, seven electrons in Orbit 2.

6. Draw a BohrRutherford diagram for each of the following molecules
1 Ry = e4me 8ϵ2 0h2 = 2.18 × 10 − 18 J. and this simplifies the allowed energies predicted by the Bohr model (Equation 7.4.11) as. En = − (2.18 × 10 − 18)Z2 n2 J = − Z2 n2 Ry. Hence, the energy of the electron in an atom also is quantized. Equation 7.4.12 gives the energies of the electronic states of the hydrogen atom.

Fluorine Bohr Model
Example 2: Fluorine This is the Bohr -Rutherford Diagram for Fluorine (Atomic Number 9). Fluorine is the ninth element of the Periodic Table. As shown, Fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). Thus, its Atomic Mass is 19. Fluorine also has nine total electrons -- two electrons in Orbit 1, seven electrons in Orbit 2 .
Fluorine Bohr Model
In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons.